
Clinical Trial
Our well qualified team will not only help in creating strategic clinical development plans as well as providing tactical support to clients:
- Our Qualified and knowledgeable clinical writers will plan and execute clinical evaluation, meeting the regulation and guidelines as per the manufacturers’ requirements;
- Adopt in various software tools enabling unmistakable, organized and efficient way of documentation;
- Specialist in literature search and appraisal, interpretation and analysis of data;
- Meticulous effort towards timely project completion;
- Creates well-structured procedure, SOPs, and associated templates;
- Guides and supports clients on timely CER updates;
What Medra+ Does:
- Our Team develops project plans which includes logistics of pre-study feasibility surveys. creation, distribution and data collection
- Pre-study feasibility surveys to identify interested sites and confirm their ability to successfully participate in a given trial
- Constant interaction between our teams and clients to address their queries and resolve them at the earliest
- Well-Knit Medical Affairs and Project Management team for one on one interaction with client
We are the solution providers for our clients right from draft initiation till report preparation
- Clinical evaluation protocol
- Clinical evaluation report
- Collection of clinical data
- Systematic Literature Search
- IRB and DSRB submissions
- Consent Form and Patient Information Form
- PMS
- PMCF
- Predicative device comparison analysis
- CER GAP Analysis Checklist
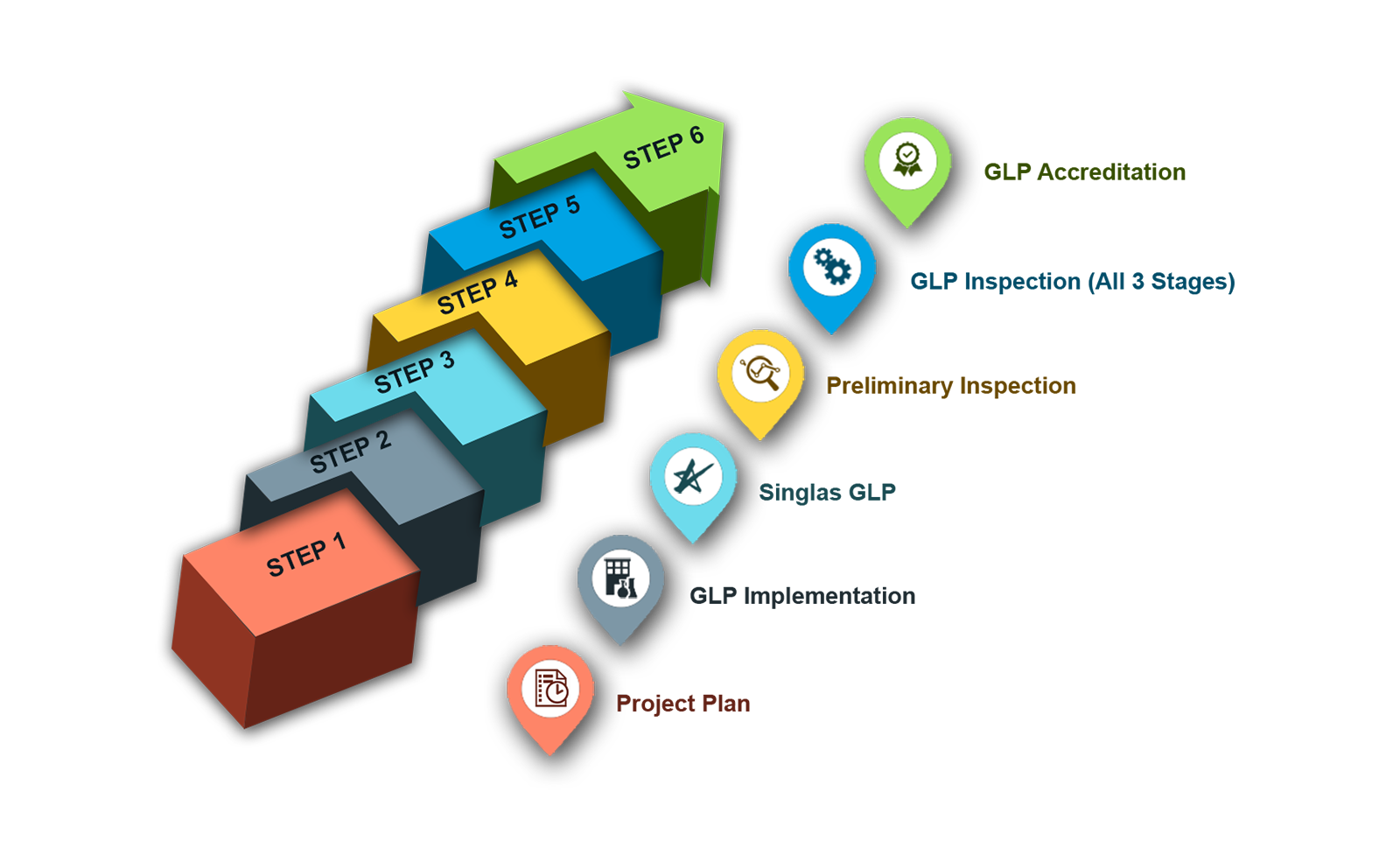
- Project plan
- GLP Implementation
- Personnel
- Facility
- Facility Update
- Test and control articles
- Singlas GLP
- Preliminary inspection
- GLP inspection (all 3 stages)
- GLP Accreditation
- Assist our client in conducting the PMS
- Supports in the framework of PMS Plan
- Bolsters in the functioning of PMS Activities
- Initiates well structured PMS plan and procedures followed by report templates
- Analyses logical PMCF requirements from the PMS data available
- Chalking out final suitable conclusions on case to case basis
PMCF process will be initiated from our team by helping the clients in collection of market information from distributors, manufacturers and end users.
Will assist in collecting existing AE, FSCA and Recall of predicate devices from major countries using their medical device authority sites such as FDA, MDA, HSA, TGA, MHLW etc. and to conclude that the client product is safer than the existing product available.
If any reportable adverse events occurs, our team will guide the client to notify the respective authority of the country in complaint registration process.
Will help in preparing risk management report by portraying the relevant parts of the clinical evaluation report as a reference tool.
We help our client in the final analysis process of the Post Market Clinical Follow up and record the results in the Clinical Evaluation Report and the technical documentation.
- Internal system audits
- Regulatory document audits
- Staff training
- Management of a Corrective and Preventive Action (CAPA) program
Contact us for 1-hour free consultation
Just submit your contact details and we’ll be in touch shortly.

